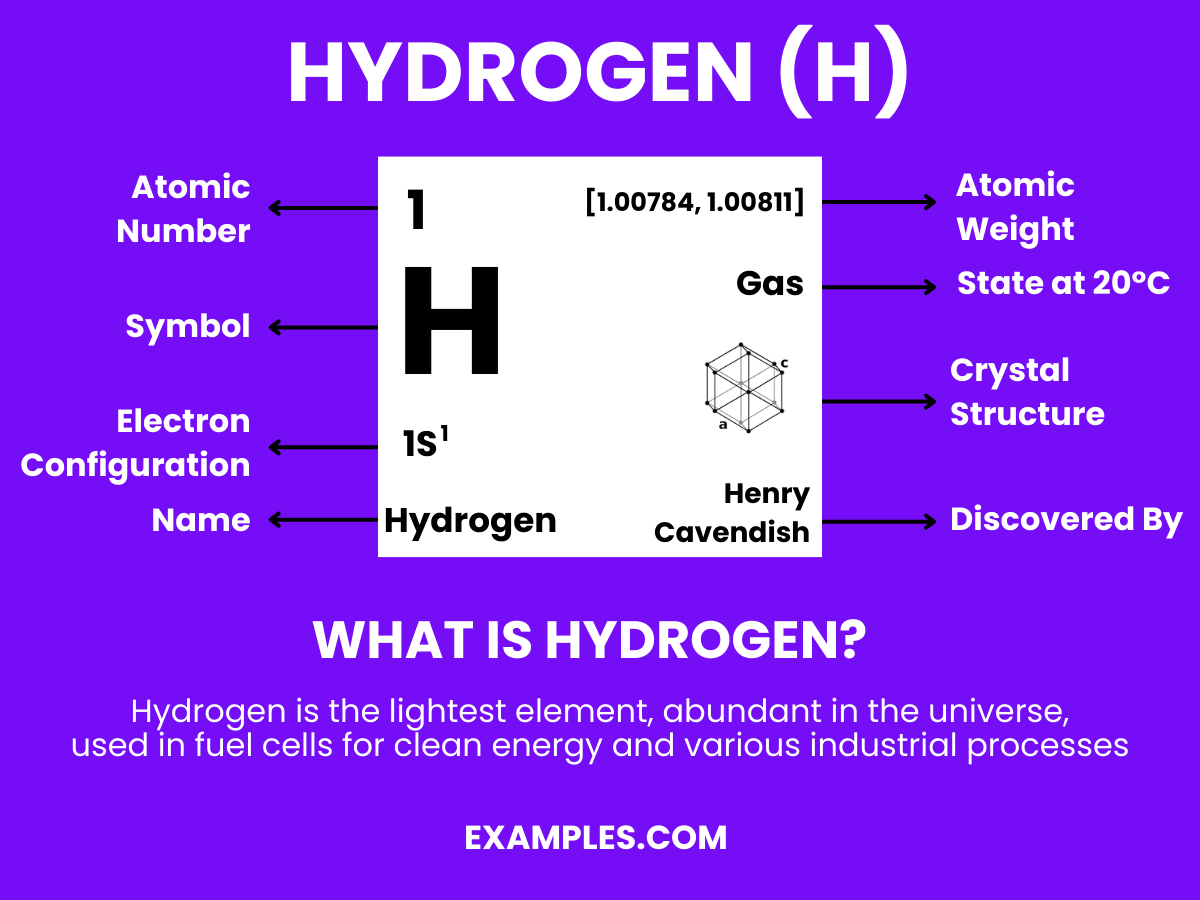

Dive into our comprehensive guide to understand Hydrogen, the most abundant element in the universe. This guide offers detailed insights and practical examples of Hydrogen’s role in water formation, fuel technology, and the vast cosmos. Learn about its isotopes, properties, and applications that impact everything from the water we drink to the stars above us. With easy-to-understand examples, discover why Hydrogen is a fundamental building block in chemistry and beyond. Embrace the world of Hydrogen today!
Hydrogen is the simplest and most abundant element in the universe, consisting of just one proton and one electron. This lightweight, colorless gas is found in great abundance in stars and gas giants and is a fundamental building block in the chemistry of life. As a versatile energy carrier with the highest energy content of any common fuel by weight, hydrogen holds promise in various fields, including renewable energy and space exploration. It’s essential in teaching the foundations of chemical reactions, bonding, and energy transformations, providing a gateway to understanding more complex scientific concepts.
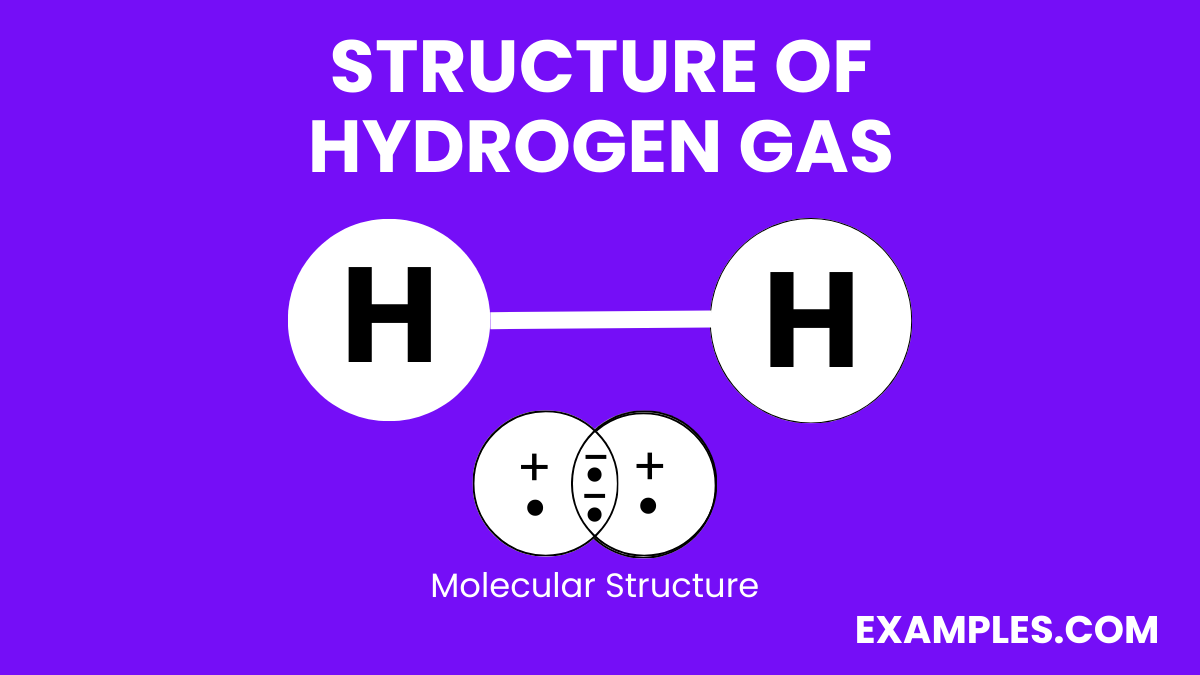
Hydrogen gas (H₂) consists of two hydrogen atoms bonded together. Each hydrogen atom has one proton in its nucleus and one electron. In the hydrogen molecule, the two atoms are bound by a covalent bond, where they share their single electrons. This sharing allows both hydrogen atoms to achieve a stable electron configuration similar to that of the noble gas Helium.
Here’s a simple representation of the structure:
The bond between the hydrogen atoms is relatively strong, resulting in a stable diatomic molecule. At room temperature and standard atmospheric pressure, hydrogen gas is a colorless, odorless, and highly flammable diatomic gas.
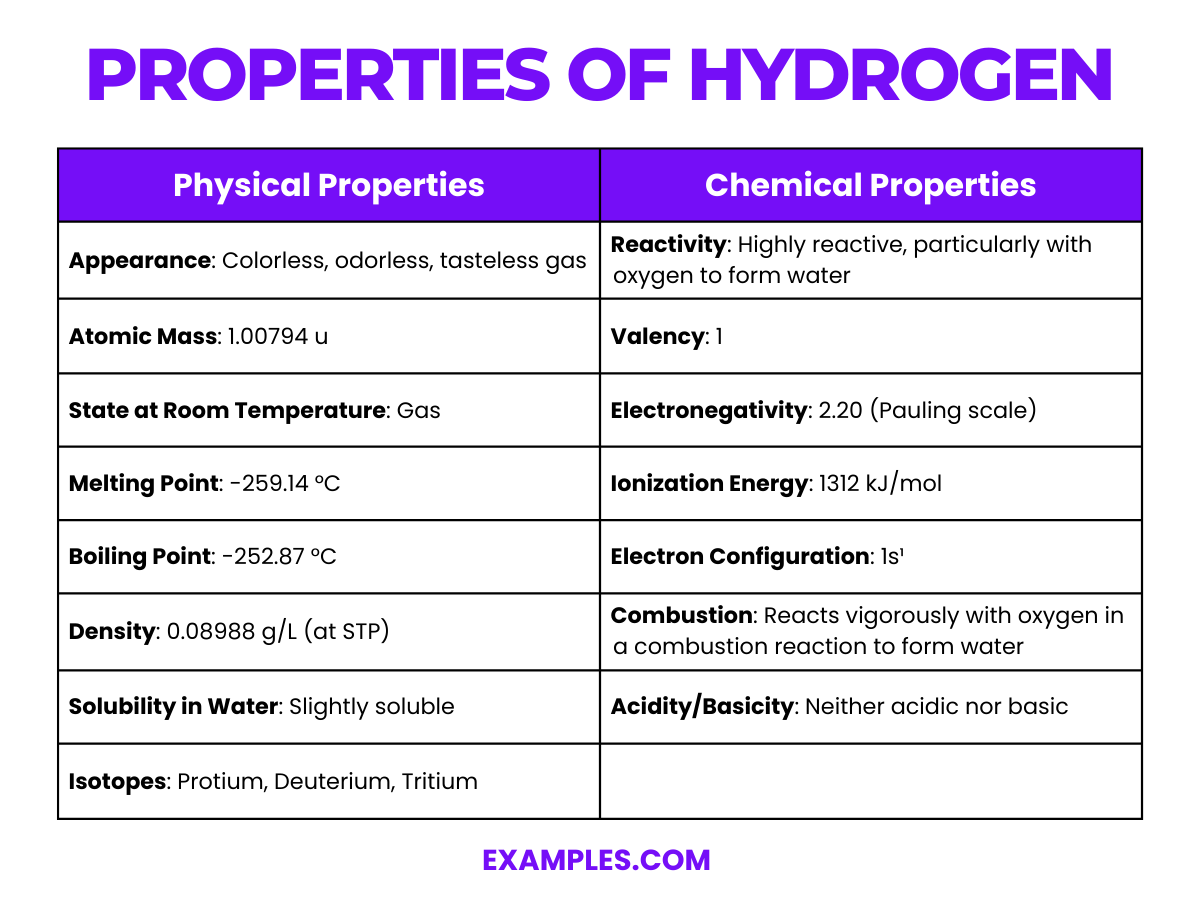
When teaching about hydrogen, it’s beneficial to compare its physical and chemical properties side by side. This approach aids in illustrating how these properties interrelate and affect hydrogen’s behavior and reactivity. Below is a detailed table for educators to use as a resource when teaching about hydrogen.
| Aspect | Detail |
|---|---|
| Chemical Symbol | H |
| Atomic Number | 1 |
| Atomic Mass | Approximately 1.008 u (unified atomic mass units) |
| State at Room Temperature | Gas |
| Color | Colorless |
| Odor | Odorless |
| Taste | Tasteless |
| Boiling Point | -252.87 °C |
| Melting Point | -259.14 °C |
| Density (at STP) | 0.08988 g/L |
| Electron Configuration | 1s¹ |
| Oxidation States | -1, +1 |
| Isotopes | Protium (¹H), Deuterium (²H), Tritium (³H) |
| Natural Occurrence | Most abundant element in the universe, primarily in stars and gas giant planets |
| Uses | In fuel cells, as a reducing agent in chemical processes, in hydrogenation of fats and oils, in welding, and as a rocket fuel when combined with oxygen |
Hydrogen is a unique element with distinct properties that make it vital in various chemical reactions and applications. Here are some of the key chemical properties of hydrogen:
Hydrogen is highly reactive. It combines with almost all elements to form binary compounds called hydrides.
Hydrogen can undergo oxidation as well as reduction. It ca-n lose an electron to form H+ (proton) or gain an electron to form Hˉ (hydride ion).
Hydrogen is known for forming acids when combined with non-metals like chlorine, sulfur, and oxygen.
Hydrogen is often used as a catalyst in various industrial chemical reactions, such as hydrogenation where unsaturated bonds in organic compounds are converted to saturated by adding hydrogen.
Hydrogen forms hydrides with almost all elements. These hydrides are categorized into ionic, covalent, and metallic hydrides.
At standard conditions, hydrogen exists as a diatomic molecule (H₂), meaning it consists of two hydrogen atoms bonded together, making it more stable.
Hydrogen has high energy content per mass and can release substantial energy when combined with oxygen in a fuel cell to produce electricity.
Hydrogen has three isotopes, Protium (¹H), Deuterium (²H), and Tritium (³H), each with unique properties affecting its chemical behavior.
| Property | Value and Units |
|---|---|
| Molecular Weight | 2.016 g/mol |
| Boiling Point | -252.87°C or -423.17°F |
| Melting Point | -259.14°C or -434.45°F |
| Density (at 0°C, 1 atm) | 0.08988 g/L |
| Specific Heat Capacity (Cp) | 14.304 J/(mol·K) |
| Heat of Formation (ΔHf°) | 0 kJ/mol (standard state) |
| Heat of Combustion | -285.83 kJ/mol |
| Standard Entropy (S°) | 130.68 J/(mol·K) |
| Critical Temperature | -239.95°C or -399.91°F |
| Critical Pressure | 1.2958 MPa or 12.8 atm |
| Property | Value |
|---|---|
| Electronegativity (Pauling Scale) | 2.20 |
| Ionization Energy | 1312.0 kJ/mol (first ionization) |
| Conductivity | Poor conductor in molecular form |
| Magnetic Susceptibility | Diamagnetic |
| Property | Value |
|---|---|
| Isotopes | Protium (¹H), Deuterium (²H, D), Tritium (³H, T) |
| Nuclear Spin | ¹H: 1/2, ²H: 1, ³H: 1/2 |
| Natural Abundance | ¹H: 99.98%, ²H: 0.02%, ³H: Trace |
| Half-life of ³H (Tritium) | 12.32 years |
| Neutron Cross Section | ¹H: Low, ²H (D): High for neutrons |
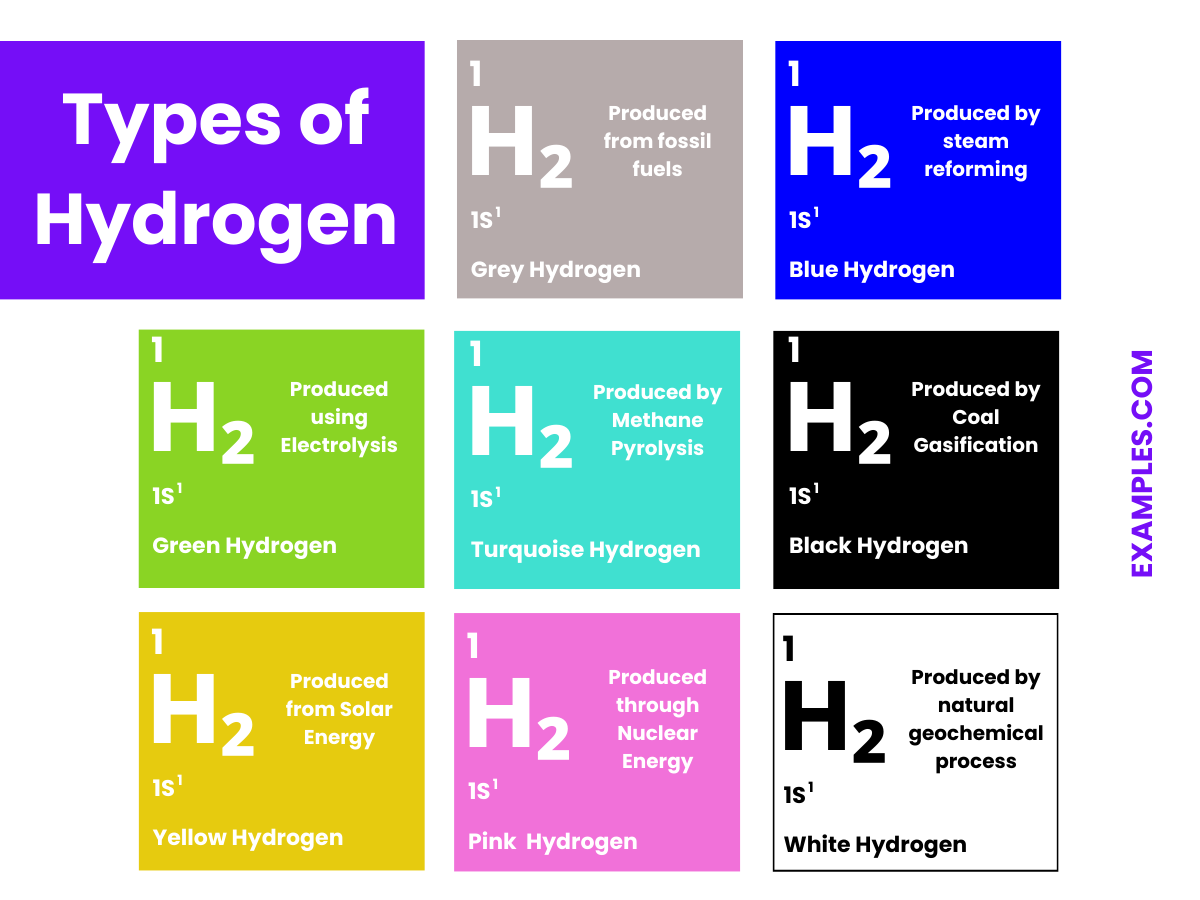
The types of hydrogen are often categorized by the method of their production, particularly focusing on the amount of carbon emissions associated with their generation. Here are the types you’ve mentioned:
These color-coded categories help distinguish the methods and environmental impacts of hydrogen production. As the world looks towards cleaner energy solutions, the focus is increasingly on green and blue hydrogen, with ongoing research to make their production more efficient and economically viable.
Dihydrogen, commonly known as molecular hydrogen or simply hydrogen gas, is a fundamental chemical widely used in various industrial and scientific applications. Teachers can utilize this information to enhance their students’ understanding of chemical reactions, stoichiometry, and industrial applications. Here, we discuss several methods of dihydrogen preparation, each accompanied by corresponding chemical equations.
Electrolysis of water is a popular method in laboratories to produce dihydrogen. The process involves passing an electric current through water to decompose it into oxygen and hydrogen gas.
Certain metals, such as zinc and iron, react with dilute acids to release dihydrogen gas. This method is often demonstrated in educational settings due to its straightforward procedure.
Chemical Equations
Passing steam over heated metals like iron, magnesium, or zinc can produce hydrogen gas. This method is significant in industrial processes.
Chemical Equations
Hydrocarbons like methane can be decomposed thermally or catalytically to produce hydrogen and other byproducts, primarily in industrial settings.
Alkali metals and alkaline earth metals react vigorously with water to form hydrogen gas and the respective hydroxide.
Chemical Equations
While discussing these methods, it’s imperative to emphasize safety precautions, especially when handling acids, alkali metals, or high temperatures. Also, consider discussing the efficiency and applicability of each method in industrial versus laboratory settings.
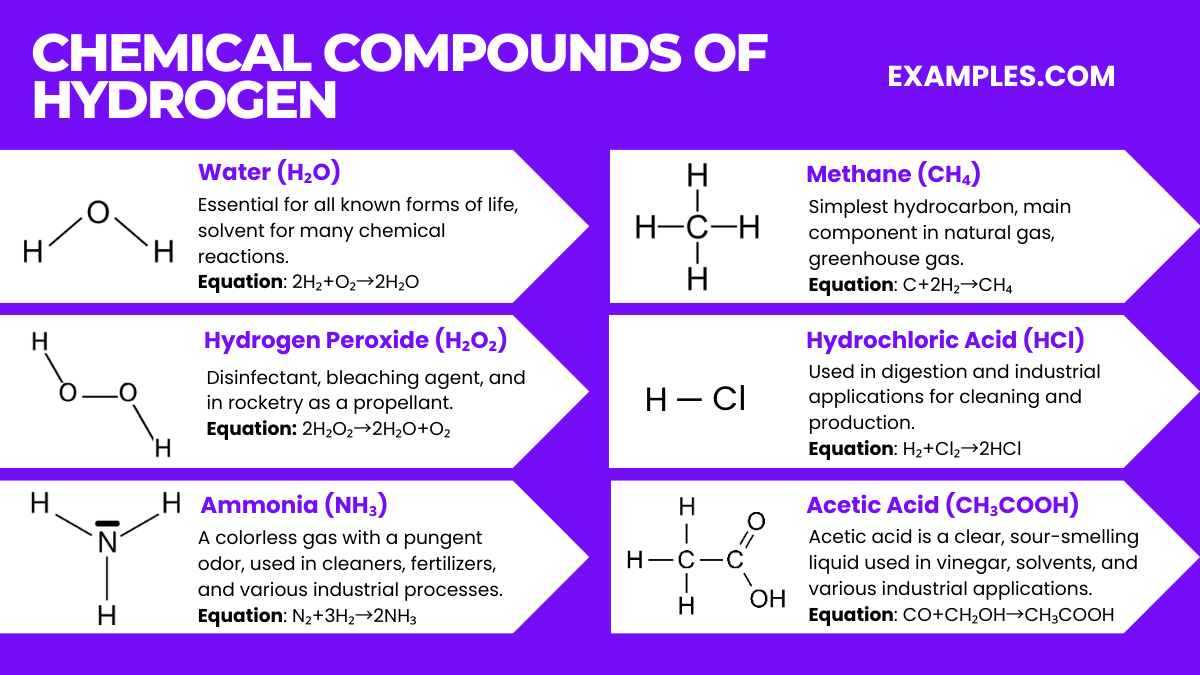
Hydrogen is known for its versatility and reactivity, forming compounds with almost all elements. Here are some of the most common and significant chemical compounds of hydrogen:
Hydrogen, the simplest and most abundant element in the universe, plays a vital role in chemistry, physics, and several industries. Understanding its isotopes is crucial for teachers educating the next generation of scientists and students delving into nuclear chemistry and physics. Here, we break down the isotopes of hydrogen for an educational audience, aiming to provide a comprehensive, engaging, and informative guide.
| Isotope | Symbol | Protons | Neutrons | Natural Abundance | Atomic Mass | Common Uses |
|---|---|---|---|---|---|---|
| Protium | ¹H | 1 | 0 | ~99.98% | 1 | Water and organic compounds |
| Deuterium | ²H or D | 1 | 1 | ~0.02% | 2 | Nuclear reactors, tracing studies, heavy water |
| Tritium | ³H or T | 1 | 2 | Extremely rare | 3 | Nuclear fusion, self-luminous paints and watches |
Protium is the most common hydrogen isotope, with an atomic mass of 1. It has one proton and no neutrons. This isotope is prevalent in the universe, making up about 99.98% of all hydrogen. It is the primary component in water and organic compounds.
In Water Formation: 2H₂+O₂→2H₂O
Deuterium, also known as heavy hydrogen, contains one proton and one neutron, giving it an atomic mass of 2. Despite its low natural abundance, it has significant scientific and industrial applications, including nuclear reactors and tracing studies in biochemistry and environmental science. Deuterium is also used in heavy water moderated reactors.
In Heavy Water: 2D₂+O₂→2D₂O
Tritium is the least common isotope of hydrogen, with one proton and two neutrons, resulting in an atomic mass of 3. It is radioactive and decays into helium-3 through beta decay with a half-life of approximately 12.32 years. Tritium is used in nuclear fusion reactions, as well as in self-luminous paints and watches.
Hydrogen, the simplest and most abundant element in the universe, serves as a fundamental building block in chemistry. Its applications are vast and varied, touching upon numerous aspects of scientific, industrial, and environmental sectors. Below, we explore its diverse uses, aimed at empowering educators with knowledge to enrich their teaching endeavors.
Hydrogen as a Clean Energy Source: Hydrogen is at the forefront of clean energy solutions. When burned or used in fuel cells, it produces only water as a byproduct, making it an attractive option for reducing greenhouse gas emissions and combating climate change. Educators can highlight the role of hydrogen in:
Chemical Industry: Hydrogen in Industrial Applications is a key reactant in numerous chemical processes, including:
Refining: Hydrogen is employed to refine petroleum, helping to remove sulfur and impurities from crude oil, and in the process, enhancing the quality of fuels.
As a Reducing Agent: In chemical reactions, hydrogen serves as a powerful reducing agent, facilitating the study and synthesis of various compounds.
Space Exploration: Hydrogen fuel has powered space shuttles and missions, demonstrating its pivotal role in propelling rockets and providing water and power in space stations through fuel cells.
Carbon Capture and Storage: Hydrogen can play a role in reducing industrial carbon dioxide emissions by reacting with CO2 to produce methane or methanol, thereby aiding in carbon capture technologies.
Pollution Reduction: By replacing fossil fuels in transportation and energy production, hydrogen significantly cuts down on air pollution, a point of discussion in environmental education.
In the medical field, hydrogen has emerging applications, such as in antioxidant therapies. Hydrogen-rich water is being studied for its potential to reduce oxidative stress, a factor in various diseases.
Hydrogen is a versatile element with various applications, influencing multiple sectors including transportation, industry, and energy. Understanding its production and utilization is crucial for educators and students alike.
Hydrogen can be produced through several methods, each with its unique technology and implications. The most common methods include:
Hydrogen has a wide range of applications in various industries. Key applications include:
Understanding the distinction between blue hydrogen and green hydrogen is vital for educators, as it influences both environmental impact and technological investment. The table below outlines the key differences:
| Feature | Blue Hydrogen | Green Hydrogen |
|---|---|---|
| Definition | Produced from natural gas through steam methane reforming. The carbon emissions are captured and stored or reused. | Produced via electrolysis of water using renewable energy sources like wind or solar. |
| Carbon Footprint | Low, as most of the CO2 is captured and stored. However, it is not completely emission-free. | Minimal to none, as it relies on renewable energy sources and water. |
| Cost | Currently more cost-effective due to the established infrastructure and technology. | More expensive due to the cost of renewable energy and electrolyzers, but expected to decrease as technology advances. |
| Use Cases | Used in industries where carbon capture and storage are feasible, like chemical production or power generation. | Ideal for sectors aiming for zero emissions, including transportation and energy sectors seeking sustainable solutions. |
Hydrogen’s high cost is due to its extraction methods, requiring significant energy for electrolysis or reforming natural gas, and its storage and transport complexities.
Hydrogen is an excellent fuel; it’s clean-burning, producing only water as a byproduct, highly efficient in fuel cells, and renewable when produced from water.
Hydrogen gas is powerful, holding the highest energy per mass among all fuels, which makes it highly efficient but challenging to store and handle safely.
Hydrogen is primarily a gas at standard conditions, the lightest and most abundant element in the universe, though it exhibits metallic properties under extreme pressure.
We write H2 instead of H because hydrogen naturally exists as a diatomic molecule, meaning two hydrogen atoms bond together to form H2, the most stable form of hydrogen gas.
Hydrogen can exist as H (a neutral atom) or H+ (a proton). H+ denotes a hydrogen ion, which occurs when hydrogen loses its electron, resulting in a positively charged ion.
Hydrogen, the simplest and most abundant element, is pivotal in chemistry and physics. Its unique properties, like lightness and reactivity, enable diverse applications from fuel cells to fertilizers. Understanding its isotopes and compounds deepens our grasp of chemical bonds and molecular structures. Embracing hydrogen’s potential can lead to revolutionary advancements in energy and science, fostering a more sustainable future.